Evolving treatment
We now know a lot more about the natural progression of the virus and how to use different combinations of antiretroviral drugs to control viral replication and reduce the incidence of HIV related mortality and morbidity. Zidovudine (AZT) was the first antiretroviral to be approved for use in 1987. It was used initially as monotherapy, however, the virus quickly developed resistance to it and it was poorly tolerated due to the high doses used causing haematological toxicity. As more antiretroviral drugs were developed, it became apparent that the combined use of at least three different drugs known then as “Highly Active Antiretroviral Therapy or HAART” produced a better virological response and lower rates of resistance.
Following the introduction of HAART, now commonly called ART, in 1996 the numbers of AIDS diagnosis and deaths have dropped significantly and now remain at a steady but low level. Now that HIV infected patients are living longer, they are developing significant co-morbidities due to ageing, chronic HIV infection and the complications caused by the long-term use of antiretroviral drugs. Hence the challenges in treating HIV patients have shifted from an acute fatal illness to a long term chronic one with polypharmacy as a key issue.
When to start antiretroviral therapy
The decision about when to start therapy has recently changed due to overwhelming data from the “Start” study that demonstrated significant benefits in terms of long-term morbidity and mortality in patients starting ART before their CD4 count drops below 500 cells/mm3. These data have been reflected in the 2015 (2016 interim update) UK BHIVA 8 and WHO 9 treatment guidelines, both of which recommend starting ART as soon as possible after diagnosis. The BHIVA guidelines also state that individuals presenting with an AIDS-defining infection, or with a serious bacterial infection and a CD4 cell count <200cells/mm3 start ART within 2 weeks of initiation of specific OI treatment.
Up until 2015 the decision to start treatment was based upon the CD4 cell count (circa 350 cells/ mm3), the CD4 percentage plus other factors such as co-infection with hepatitis B and C.
See the BHIVA guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy for more specific details. It is also advisable to check local and regional guidelines as they may vary because of cost considerations.
The link to the WHO guidelines 2018 interim update
Classes of antiretroviral drugs
Currently there are 5 licensed classes of antiretrovirals (ARVs) and they are categorised on the basis of where they act in the HIV replication cycle. Normally drugs from two (or sometimes three) classes are combined to improve efficacy so that HIV is targeted via several different mechanisms. The classes are:
- Nucleoside reverse transcriptase inhibitors (NRTIs)
- Non-nucleoside reverse transcriptase inhibitors (NNRTIs)
- Protease inhibitors (PIs)
- Entry/fusion inhibitors
- Integrase inhibitors (INIs)
Where antiretovirals work in the HIV life cycle
Understanding the HIV life cycle helps us to understand how and where antiretrovirals and other drugs work. The diagram below shows where each class of antiretrovirals act.
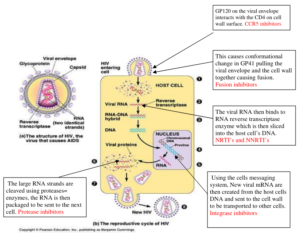
Antiretroviral drug classes
Nucleoside reverse transcriptase inhibitors (NRTIs):
The reverse transcriptase enzyme reverse-transcribes the RNA into proviral DNA. NRTIs are analogues of naturally occurring nucleosides that are needed to synthesise the proviral DNA. Once inside the CD4 cells the NRTIs are converted into their active form- the triphosphate form by a process called phosphorylation. They then compete with natural nucleosides for incorporation into the growing viral DNA chain. Once incorporated the next nucleoside cannot form a bond which results in chain termination. If HIV cannot convert RNA into DNA, it cannot enter the cell nucleus nor continue replication.
NRTIs are often referred to as the ‘backbone’ of a combination and most patients starting therapy will have at least 2 drugs from this class in their regime.
Currently available NRTIs include:
- Abacavir (Ziagen®)
- Didanosine, or ddI (Videx®) – rarely used
- Emtricitabine or FTC (Emtriva®)
- Lamivudine or 3TC (Epivir®)
- Stavudine or d4T (Zerit®) – rarely used
- Zidovudine or AZT (Retrovir®)
Nucleotide reverse transcriptase inhibitors (NtRTIs)
This class of drug works in exactly the same way as NRTIs, with the exception that an extra phosphate is attached to the drug molecule. This means that the drug is present in a more active form, as it does not have to be phosphorylated in the same way as NRTIs do. It also may be active in more types of cells within the body. At present the only antiretroviral drug available in this class is Tenofovir. It is available in its original form as Tenofovir disoproxil and in a prodrug form called tenofovir alafenamide, commonly known as TAF. TAF is preferentially taken up into the lymphoid tissue resulting in much lower blood levels of tenofovir, should result in less side effects.
- Tenofovir disoproxil (Viread®)
- Tenofovir alafenamide (TAF)
Non-nucleoside Reverse Transcriptase Inhibitors (NNRTIs)
NNRTIs work at the same site as NRTIs but are structurally different. They block reverse transcription by binding directly to the reverse transcriptase enzyme so are not incorporated into the viral DNA like the NRTIs
Currently available NNRTIs are:
- Efavirenz (Sustiva®)
- Nevirapine (Viramune®)
- Etravirine (Intelence®)
- Rilpivirine (Edurant®)
- Doravirine (Pifeltro®)
Integrase inhibitors (INIs)
These drugs block the action of the integrase enzyme. The HIV virus uses this enzyme to enter the cell’s nucleus. Once inside the nucleus, viral DNA is integrated into the host chromosomal DNA producing new chains of HIV proteins (mRNA).
Currently available integrase inhibitors are:
- Raltegravir (Isentress®)
- Elvitegravir (Only available as a combination tablet in Stribild® and Genvoya®)
- Dolutegravir (Tivicay®)
- Bictegravir (Only available as a combination tablet in Biktarvy®)
Protease inhibitors (PIs)
Protease is the third HIV enzyme; its role is to break up the long chains of HIV proteins into smaller pieces that can be assembled to make new viruses. Protease inhibitors prevent protease from cutting the protein chains into the shorter pieces to make new virus particles. Most PIs need to be boosted with a low dose of ritonavir or cobicistat that gives a more even pharmacokinetic profile (AUC) which ensures sufficient levels of drug in the body and minimises side effects. Currently available PIs are:
- Atazanavir (Reyataz®)
- Darunavir (Prezista®)
- Indinavir (Crixivan®) – rarely used
- Fosamprenavir (Telzir®) – rarely used
- Nelfinavir (Viracept®) – no longer used
- Lopinavir and ritonavir (Kaletra®)
- Ritonavir (Norvir®) – only used as a booster at a low dose
- Saquinavir (hard gel capsules or tablets: Invirase®) – no longer used
- Tipranavir (Aptivus®) – rarely used
Cobicistat is a pharmacokinetic enhancer that does not have any antiviral effect of its own.
Entry/Fusion Inhibitors
Entry or Fusion inhibitors work by attaching themselves to proteins on the surface of CD4 cells (entry inhibitor) or on the surface of the virus (fusion inhibitor) thereby blocking HIV from entering the cell.
The fusion inhibitor Enfuvirtide (Fuzeon®), also known as T20 is the only available drug that works by binding itself to the virus surface. T20 is manufactured only as an injection and is usually used for people who have extensive drug resistance because of the inconvenience of twice daily injections.
The entry inhibitor Maraviroc (Celsentri®) is commonly called a CCR5 inhibitor and works by binding itself to the CCR5 protein on the surface of CD4 cells. These CCR5 receptors are primary entry points for the HIV virus, particularly in early stages of infection. Not all HIV binds to a host CD4 cell via the CCR5 receptor (some use CXCR4 receptors) so it is essential to first carry out a genetic test called a trofile assay (also known as a tropism test) to determine if a CCR5 inhibitor would be appropriate to use.
Antiretrovirals – generic and brand names, abbreviations and doses
Combination formulations
Summary of antiretroviral drugs, side effects, monitoring and counselling points
What to start treatment with
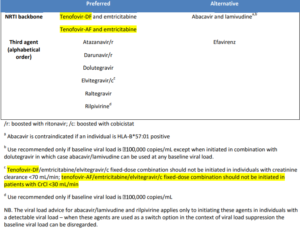
There are several options for first line therapy and the BHIVA treatment guidelines8 recommend that therapy-naïve patients start an ART regimen that contains:
(1) Two nucleos(t)ide reverse transcriptase inhibitors (NRTIs) with tenofovir and emtricitabine as the preferred option and abacavir and lamivudine as an acceptable alternative though it should be used with caution in patients with VL> 100,000 (though this is not applicable when it is used with dolutegravir)
(2) Plus one of the following:
- The NNRTI: Rilpivirine
- The boosted PIs (PI/r), Atazanavir/r and Darunavir/r
- The INIs, Dolutegravir, Raltegravir and Elvitegravir/c
- Efavirenz is suggested as an alternative 3rd agent
As a novel ARV strategy where there is need to avoid abacavir or and tenofovir, BHIVA guidelines suggests the use of a dual based ART regimen of darunavir/r with raltegravir in treatment naïve patients with CD4 count > 200 cells/mm3 and viral load <100,000.
The exact regimen choice is patient specific and multi-factorial, including specific haematological and biochemical results such as VL, CD4 cell count, renal and hepatic function and genetic markers such as HLA status. See the table below for specific limitations with certain ARVs. The presence of hepatitis, tuberculosis, neurocognitive impairment, and cardiovascular risk or other co-morbidities plus drug-drug interactions (DDIs) with current or future medication must also be considered. Patient preference for particular medications or formulations and specific toxicity concerns will also be taken into account when choosing a regimen
Some patients may have transmitted or primary resistance, meaning that the virus has genetic mutations associated with ARV resistance despite the patient never being exposed to any treatment. The prevalence of primary drug-resistant in the UK is around 11%7. BHIVA guidelines recommend that resistance testing is undertaken for all newly diagnosed patients as the results may affect drug choice. These patients may not be able to use one of the first line options and may require a tailor-made regimen. The BHIVA treatment guidelines of 2015 had an interim update in 2016 and those changes are highlighted in yellow in the table below which shows the preferred and alternative options in naïve patients. Further updates to the treatment guidelines are due soon.
Table of BHIVA summary recommendations for choice of ART in naïve patients8
Monitoring treatment
BHIVA recommends that patients are assessed at baseline, within two to four weeks of starting treatment, and then, three to six monthly thereafter (unless management is problematic)8
The following haematological and biochemical tests should be undertaken to assess treatment response and monitor for toxicity:
- Viral load
- CD4 cell count
- Full blood count
- Creatinine
- Estimated glomerular filtration rate
- Liver function tests
- Glucose
- Bone profile
Effective ART for treatment-naive patients should achieve viral suppression, demonstrated by an undetectable viral load within 12 to 24 weeks of commencing therapy. If viral decline is slow, then adherence should be checked along with other possible reasons for poor response such as drug interactions or resistance. Patients can be described as being stable on therapy when they have maintained viral suppression (<50 copies/ml) for over 6 months. Thereafter patients are usually seen by their doctors every 4-6 months and in certain clinics stable patients are able to have telephone consultations after they have had their blood results reviewed.
Side effects
There is considerable variability in the severity of side effects experienced by patients receiving ARVs, ranging from the unnoticeable to the unbearable. Patients may experience side effects early in their therapy or later as a result of long-term toxicity.
Common initial side effects of all antiretrovirals include:
- rash
- nausea
- vomiting
- headache
- diarrhoea
Abnormal liver function tests, hepatotoxicity and rarely, Stevens-Johnson syndrome can also occur in the early stages of therapy.
Additionally, efavirenz, dolutegravir and bictegravir can cause central nervous system side effects, such as:
- dizziness
- vivid dreams
- insomnia
- morning drowsiness
- depression
Protease inhibitors are known to cause gastrointestinal disturbances, which can often be managed using appropriate medicines such as anti-emetics and loperamide. Side effects often coincide with peak drug levels in the blood and can be more intense during the first few days of treatment until a steady state is achieved. Changing the timing of doses or taking them with or without food can often help minimise effects. Long-term side effects include reduced renal function and bone mineral density, dyslipidaemia, diabetes and changes to glucose control.
Toxicity issues such as peripheral neuropathy, lipodystrophy and changes to body shape are associated with the older antiretrovirals and are now rarely seen in practice. These older side effects are often the ones patients will have concerns about when they start treatment so it is important to reassure them that the newer ARVs do not cause these body shape changes.
There are ways to manage some of these longer-term toxicities. For example, metabolic syndrome (dyslipidaemia and diabetes) can be managed pharmacologically using lipid-lowering medicines and anti-diabetic treatment, and through lifestyle interventions such as smoking cessation, diet modifications and encouraging exercise.
Interactions
Treatment of patients with HIV is complicated by drug interactions. As people who are infected with HIV are now living longer and there are a high proportion of patients over 50 years of age, there is a greater likelihood of them developing co–morbidities that require medication which may have interactions with the ARVs. NNRTIs and protease inhibitors have the greatest potential to interact, both with each other and with other drugs. Often dosage adjustments or sometimes regimen changes are necessary. Some of the most common interactions occur between drugs metabolised by CYP450 in particular the CYP450 3A4 enzyme. NNRTIs and PIs are both substrates for CYP3A4 and they can also act as enzyme inducers, inhibitors or both and can therefore affect the clearance of other drugs metabolised via the same pathway. Other enzymes and transport proteins such as p-glycoproteins and UGT are also involved in ARV metabolism and are also affected by ARVs. Drug interactions with ARVs can have serious consequences as low levels of ARVs can lead to resistance and high levels can result in toxicities.
As there are many possible interactions between ARVs and non ARVs, in addition to the fact that patients receive medicines from multiple sources, it is vitally important to do a thorough drug history to establish if they are taking anything that may interact with their ARVs. This includes all medicines prescribed by their GP, bought OTC medicines, inhalers, nasal sprays, creams, injections (including single dose), herbal remedies, recreational drugs and supplements. It is prudent to prompt the patient with these categories as many do not consider inhalers as a medicine and may omit it from their list.
Also due to the increased co-morbidities, there may be many different doctors and specialties involved in the patient’s care so it is important to educate the patient to inform the clinic (ideally the pharmacist) each time they are given a new medication including injections such as midazolam for procedures etc. It is unlikely that the other specialties will be aware of the drug interactions so the patient needs to be the link between them and the clinic.
Some interactions are well-documented and dosing recommendations are available. Other interactions are less easy to predict. In these cases, it is necessary to go back to first principles and use pharmacokinetic and pharmacodynamic information to understand possible mechanisms of interactions. Always consult a specialist HIV pharmacist if there are any doubts or queries regarding interactions.
These are some general points to consider:
- Atazanavir and rilpivirine require an acid environment for absorption; therefore, proton pump inhibitors should not be used. H2-receptor antagonists and antacids can be used with caution only if the timing of doses is adjusted according to their summaries of product characteristics. See the drug summary table above or the drug sheets for each drug’s specific requirements.
- Medicines that contain iron, magnesium and aluminium (i.e. medicines that contain polyvalent cations) such as multivitamins and minerals and indigestion remedies interact with integrase inhibitors, reducing their absorption. They should either be avoided or dose adjusted. See the drug summary table above or the drug sheets for each drug’s specific requirements.
- Interactions can occur with medicines administered by other routes that would not usually be anticipated to lead to a reaction. For example, concentrations of nasal, inhaled and intra-articular corticosteroids can be raised by protease inhibitor-based regimens, causing corticosteroid toxicity (there have been many reports of Cushing’s syndrome caused by this interaction)
- Different medicines within the same class can have different interaction profiles. For example, protease inhibitors and cobicistat can cause significant increases in simvastatin levels (approximately 600%), as a result of CYP3A4 inhibition, and concomitant use is contraindicated. However, atorvastatin — which is not solely metabolised by CYP3A4 — can be used with a dose adjustment
Sources of interaction data can be found in the SPCs of the relevant drugs and on the following websites.
Commonly known as the Liverpool website: www.hiv-druginteractions.org
The Toronto HIV drug interaction websites: www.hivclinic.ca/main/drugs_interact.html
Drug-drug Interactions between ARVs and Anti-Psychotics10
Drug-drug interactions between corticosteroids and ARVs10
Boosted Atazanavir & proton pump inhibitors
|
Coadministration of omeprazole (40 mg once daily) with atazanavir and ritonavir (300/100 mg once daily) resulted in a substantial reduction in atazanavir exposure (approximately 75% decrease in AUC, Cmax, and Cmin). Coadministration of omeprazole (20 mg once daily) with an increased dose of atazanavir and ritonavir (400/100 mg once daily) in healthy volunteers resulted in a decrease of approximately 30% in the AUC, Cmax and Cmin of atazanavir relative to atazanavir and ritonavir (300/100 mg once daily) without omeprazole. In practice tend to avoid PPI co-administration with atazanavir. |
Ritonavir-containing regimens and statins
|
Atorvastatin – Use with caution. The risk of myopathy including rhabdomyolysis may be increased when HIV protease inhibitors, including ritonavir, are used in combination with atorvastatin. Titrate atorvastatin dose and use the lowest possible dose with careful monitoring. HMG-CoA reductase inhibitors which are highly dependent on CYP3A metabolism, such as lovastatin and simvastatin, are expected to have markedly increased plasma concentrations when co-administered with ritonavir dosed as an antiretroviral agent or as a pharmacokinetic enhancer. Since increased concentrations of lovastatin and simvastatin may predispose patients to myopathies, including rhabdomyolysis, the combination of these medicinal products with ritonavir is contraindicated. The metabolism of fluvastatin and pravastatin is not dependent on CYP3A, and interactions are not expected with ritonavir. If treatment with a statin is indicated, pravastatin or fluvastatin are recommended |
Not all interactions are bad: a useful interaction that is widely exploited is when ritonavir and cobicistat are dosed with some protease inhibitors. Ritonavir and cobicistat are potent inhibitors of CYP3A4 which metabolises most of the PIs; hence the beneficial interaction enables a lower dose to be used and produces a smoother pharmacokinetic profile with more consistent drug levels and fewer side effects. PI combinations involving this interaction are commonly known as ‘boosted’ PIs.
If necessary therapeutic drug monitoring (TDM) can be used to ensure adequate levels of an ARV is maintained if an interaction is suspected. TDM is commercially available for NNRTIs, PIs and INIs but not for NRTIs as their active drug is within the cell so it is more difficult to measure and this is usually only undertaken in the setting of a clinical trial.
See also HIVPA study session on drug-drug interactions which can be accessed via eHIVe.
Adherence and resistance
Adherence means taking medication in accordance with the prescribed instructions. This involves the correct amount of drug at the correct time and in the correct way, taking into account food restrictions and requirements. To maintain viral suppression ARVs must be taken at the same time every day and according to the dietary restrictions. This means every 24 hours for a once daily regimen and every 12 hours for a twice daily regimen. Patients need to have an adherence rate of 95% to reduce the chance of resistance i.e. if patients are taking a once daily treatment, 95% adherence means missing no more than one dose a month. If patients are taking twice a day, 95% adherence means missing no more than two doses a month. When ARVs are not taken at the same time there will be intervals when there will be subtherapeutic drug levels in the body which can lead to drug resistance.
HIV treatment is lifelong and adhering to therapy is essential for sustaining viral suppression. Advances in drug development have led to newer medicines that are better tolerated and to regimens that are more convenient for patients. However, patients can struggle to adhere to their regimen for many reasons. These include:
- Failure to accept HIV-positive status and subsequently the need for therapy
- Lack of understanding of the benefits of ART, especially if non-symptomatic
- Perceived adverse impact on lifestyle (food requirements, strict timings of medicines, etc.)
- Fear of disclosing HIV status to others
- Fear of side effects and of developing stigmatising side effects (e.g., lipodystrophy)
- Perceived harm from taking antiretrovirals
- Intolerance to side effects
- Misunderstanding how to take doses
- Forgetting to take medicine
Adherence can be improved by good counselling and providing support for patients around taking their medication.
For more information, consult counselling document and the BHIVA guidelines on adherence.
The study day talks on adherence and counselling can be accessed on eHIVe
Also available for further reading: NICE guidelines on Medicines Adherence (CG76, Jan 2009)
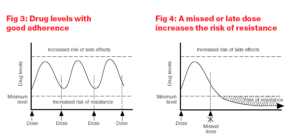
The risk of drug resistance is highest when the levels of a drug in the body are low. This usually occurs when patients miss doses, take doses erratically or stop treatment.
The latter is especially important for combinations containing medicines with differing half-lives. Levels of ARVs with longer half-lives will remain in the body after those with shorter half-lives have cleared, effectively resulting in a period of monotherapy, which can lead to the development of resistance. If there is insufficient drug in the body, the virus can mutate to escape drug attack and develop resistance to the small amount of drug that is present. Thereafter the drug resistant virus replicates and becomes stronger despite the presence of full therapeutic drug levels.
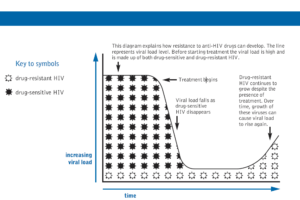
Patients can also be infected with a strain of HIV that is already resistant to some or all HIV drugs. As already stated about 11% of new infections in the UK have resistance to at least one medication. For further information on resistance please see the study day on eHIVe
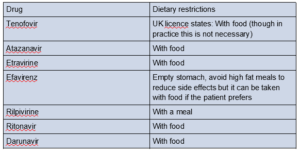
Often dietary restrictions can make taking some ARVs complicated.
The table below indicates how some ARVs should be taken with respect to food
It is an important role of the pharmacy team to ensure a patient is supported in taking their ARVs and other medication to enhance adherence and ensure a successful treatment outcome.Some patients may have difficulty swallowing some of the ARVs so below is a table of alternative formulations and how some may be crushed or dissolved.
Administration of ARVs in patients who have swallowing difficulties11
Click here for part 4